Hydrogen-Analyser
System for quick and accurate determination and analysis of the current hydrogen content in Al alloys
Controlling dissolved hydrogen in liquid aluminium alloys is a critical requirement for the aluminium industry.
Aluminium producers and foundries around the world are investing heavily to ensure that dissolved hydrogen levels remain within acceptable limits. If the hydrogen content is too high during solidification, there is a possibility of cavities / pores forming.
Conversely, a moderate hydrogen content can be desirable under certain conditions in order to positively influence the properties of the flow and mould filling capacity relevant to casting, as well as the feeding capacity and shrinkage.
As such, an optimum hydrogen content exists, which should ideally be processed in a controlled manner during casting.
Hydrogen measurement can be carried out by sampling or through in-situ measurement in the melt. IDECO therefore offers two different measuring processes.
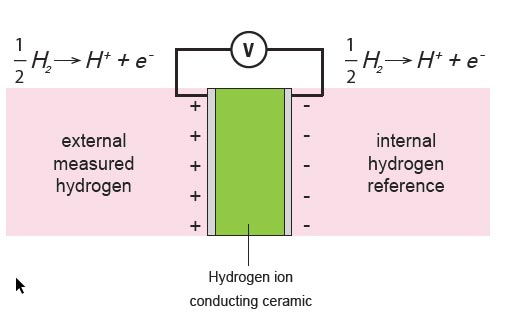
In-situ measurement using a HYCAL hydrogen measuring device from the manufacturer EMC, is based on a ceramic material (CaZrO3-In) that is able to conduct hydrogen ions at elevated temperatures. If the reference hydrogen concentration is known, a hydrogen concentration cell is used.
The sensor contains a patented solid state reference that provides a known pH2 value for a given temperature. Depending on the pH2 difference between the external and internal chamber of the sensor, voltage is generated which - in conjunction with the temperature and the hydrogen solubility of the respective alloy - enables an accurate measurement of the hydrogen to be determined.
The following schematic diagram shows how a voltage is generated on the wall of the calcium zirconate material exposed to a pH2 difference.
The IDECO® Hydrogen-Analyser is a system for quick and accurate determination and analysis of the current hydrogen content in aluminium alloys by means of sampling.
The applied measurement technique is based on the principle of the "first gas bubble" according to Y. Dardel.
To facilitate the measurement, the melt sample is poured into a crucible heated to an appropriate temperature, which can be evacuated. As soon as the ambient pressure is less than or equal to the partial pressure of the gas dissolved in the melt, the dissolved gas escapes from the melt. This is visible through the emergence of bubbles on the metal surface.
Because hydrogen is the only dissolved gas in aluminium, this process serves to determine the hydrogen partial pressure. A "real" bubble is a bubble that is followed by the emergence of further bubbles in quick succession.
The hydrogen content is calculated from the hydrogen partial pressure determined in this way, the simultaneous melt temperature, the time course of solidification and the constants. The hydrogen concentration in the melt can be calculated using Sieverts' law.
The calculation takes place as follows:
log CH = 0.5 * log pH - A / T + B
with
CH Concentration of hydrogen dissolved in aluminium in cm³/100g
pH Partial pressure of hydrogen in mbar
T Temperature of the melt in K
A, B Constants for consideration of the alloy to be tested
The constants take into account aspects including the solubility of elements such as silicon, magnesium, copper and manganese.








